Abstract
Background: Mantle cell lymphoma (MCL) is a type of non-Hodgkin lymphoma with a heterogenous clinical course and outcomes dependent on various clinical and biological prognostic factors. About 17% of MCL patients (pts) have an indolent course without the need to initiate treatment at diagnosis (Abrisqueta et al 2017). Therefore, our objective was to describe baseline characteristics of pts who received treatment more than 3 months after diagnosis (Observation) and contrast it with those who received treatment within 3 months of diagnosis (Early treatment)
Methods: Data was collected from the nationwide Flatiron Health electronic health record (EHR)-derived de-identified database. The Flatiron Health database is a longitudinal database, comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction. During the study period, the de-identified data originated from approximately 280 cancer clinics (~800 sites of care). Inclusion criteria included pts diagnosed with MCL between January 2011 to October 2021 and with a follow up of more than 1 year from the time of diagnosis with at least two visits documented in EHR. Patients who initiated any treatment within 90 days of diagnosis were the early treatment group while the remainder made up the observation group. Baseline variables including age, sex, practice type, disease subtype, Ann Arbor stage, presence of deletion of chromosome 17p, lactate dehydrogenase (LDH), WBC count, eastern co-operative oncology group (ECOG) performance status, Ki-67 and SOX11 staining were collected within 90 days of diagnosis. MIPI original score was calculated using the formula MIPI formula = [0.03535 × Age (years)] + 0.6978 (if ECOG 2-4) + [1.367 × log10(LDH/ULN)] + [0.9393 × log10(WBC)]. Kaplan Meier method was used to calculate real- world overall survival (rwOS) from the date of diagnosis and compared by log rank test between the two groups. Time to treatment initiation (TTTI) was defined as time from diagnosis to time of first treatment. Baseline characteristics between group were compared using chi square test or Kruskal-Wallis test.
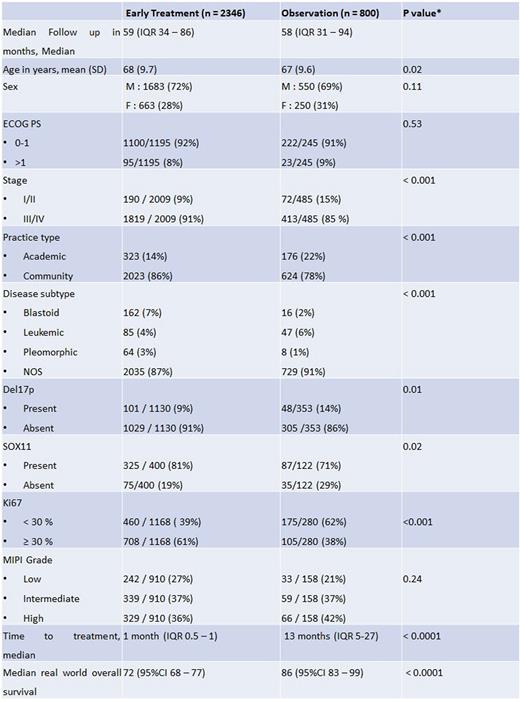
Results: Of 4336 patients, 3146 patients met inclusion criteria with 2346 patients (75%) receiving treatment within 3 months of diagnosis (early treatment group). The remaining 800 patients (25%) made up the observation group. Of these, 274 patients (34%) did not have any documented treatment. The median follow up duration for the entire group was 59 months (IQR 33 - 88).The baseline characteristics are as described in table 1. The median time to treatment initiation was 1 month (IQR 0.5 - 1.0) for the early treatment group versus 13 months (IQR 5 - 27) for the observation group. Compared with the early treatment group, the observation group was found to have a lower mean age and a higher proportion of patients with stage I/II disease (15% versus 9%), diagnosis made at an academic center (22% versus 14%), presence of deletion 17p (14 % versus 9%) and Ki67 staining < 30% (62% versus 39%). The early treatment group had higher proportions of patients with blastoid variant MCL (7% versus 2%) and SOX11 staining (81% versus 71%). The observation group showed a superior rwOS, with rwOS of 86 months (95%CI 83 - 99) for the observation group compared to 72 months (95%CI 68 - 77) for the early treatment group.
Conclusions: Our study shows that approximately 25% of all newly diagnosed MCL did not receive immediate treatment, with a median time from diagnosis to treatment of 13 months for initially observed patients. These patients were older and had early-stage disease with a low Ki-67 index. Despite delayed treatment initiation, the rwOS for these patients was superior to that of patients who received immediate treatment.
Disclosures
Narkhede:EUSA pharmaceuticals;: Research Funding; Roche: Research Funding; Gilead: Research Funding; Genmab: Research Funding; Gilead/Forty-seven: Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genetech: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seagen Inc.: Research Funding. Goyal:Sutro Biopharma: Research Funding; Viracta Therapeutics: Research Funding; SeaGen: Research Funding; UpToDate: Patents & Royalties; 2nd.MD: Consultancy. Mehta:Takeda: Research Funding; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Norvartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BeiGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; I-MAB: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche-Genentech: Research Funding; Merck: Research Funding; Kyowa Kirin: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Juno pharmaceuticals/BMS: Consultancy, Research Funding; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite/Gilead: Consultancy, Research Funding; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; fortyseven Inc./Gilead: Consultancy, Research Funding, Speakers Bureau; Celgene/BMS: Consultancy, Research Funding, Speakers Bureau; Affimed: Research Funding; Innate pharmaceuticals: Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Morphosys/Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal